Home
Products
Ganetespib (STA-9090)
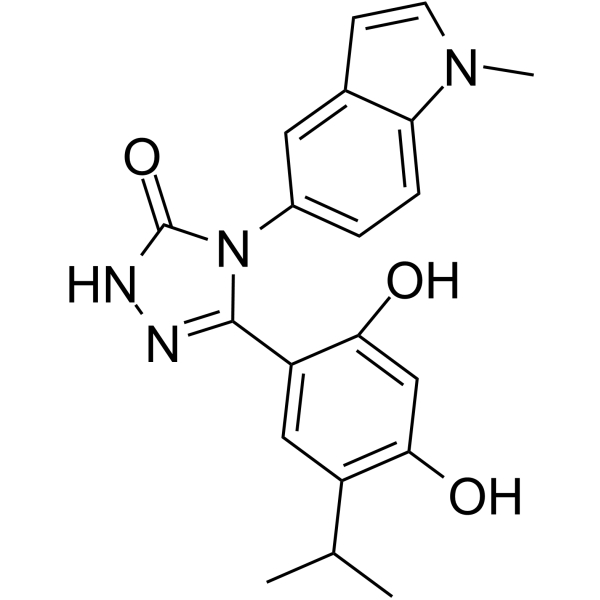


| Product Name | Ganetespib (STA-9090) |
| Price: | Inquiry |
| Catalog No.: | CN00338 |
| CAS No.: | 888216-25-9 |
| Molecular Formula: | C20H20N4O3 |
| Molecular Weight: | 364.4 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | Oc1cc(O)c(cc1c1n[nH]c(=O)n1c1ccc2c(c1)ccn2C)C(C)C |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Ganetespib is a heat shock protein 90 (HSP90) inhibitor which exhibits potent cytotoxicity in a wide variety of hematological and solid tumor cell lines. |
| Target | HSP90 |
| In Vitro | Ganetespib causes depletion of receptor tyrosine kinases, extinguishing of downstream signaling, inhibition of proliferation and induction of apoptosis with IC50 values ranging 2-30 nM in genomically-defined NSCLC cell lines. Ganetespib is also approximately 20-fold more potent in isogenic Ba/F3 pro-B cells rendered IL-3 independent by expression of EGFR and ERBB2 mutants[1]. Ganetespib exhibits potent in vitro cytotoxicity in a range of solid and hematologic tumor cell lines, induces the degradation of known Hsp90 client proteins, displays superior potency to the ansamycin inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG)[2]. Ganetespib is a potent HSP90 inhibitor, and shown to kill canine tumor cell lines in vitro[3]. Ganetespib possesses superior JAK/STAT inhibitory activity to both P6 and 17-AAG in terms of potency or duration of response in the HEL92.1.7 cells[4]. |
| In Vivo | Ganetespib (125 mg/kg, i.v.) accumulates in tumors relative to normal tissues and displays greater in vivo efficacy than 17-AAG without increased toxicity and inhibits proliferation and induces apoptosis in parallel with EGFR depletion in NCI-H1975 xenografts[1]. Ganetespib (100, 125, 150 mg/kg, i.v.) shows potent antitumor efficacy in solid and hematologic xenograft models of oncogene addiction, as evidenced by significant growth inhibition and/or regressions[2]. |
| Cell Assay | Cells are grown in 96-well plates based on optimal growth rates determined empirically for each line. Twenty-four hours after plating, cells are treated with the indicated compounds or controls for 72 hours. AlamarBlue is added (10% v/v) to the cells, and the plates are incubated for 3 hours and, then, subjected to fluorescence detection. For the comparative viability/apoptosis assay, NCI-H1975 cells are treated with escalating concentrations of ganetespib for the indicated time periods and subjected to viability analysis via CellTiter Fluor and apoptosis via Caspase Glo 3/7. |
| Animal Admin | Mice: NCI-H1975 or HCC827 cells are cultured as above and 0.5-1×107 cells are mixed with 50% RPMI 1640/50% Matrigel and subcutaneously injected into the flanks of SCID mice. For efficacy studies, animals with 100-200 mm3 tumors are then randomized into treatments groups of eight. Tumor volumes (V) are calculated by the equation V=0.5236×L×W×T (Length, width, and thickness). Animals are treated by intravenous bolus tail vein injection at 10 mL/kg with ganetespib formulated in 10/18 DRD (10% DMSO, 18% Cremophor RH 40, 3.6% dextrose and 68.4% water). As a measurement of in vivo efficacy, the relative size of treated and control tumors [(%T/C) value] is determined from the change in average tumor volumes of each drug-treated group relative to the vehicle-treated group, or itself in the case of tumor regression. Body weights are monitored daily. For biomarker studies, mice bearing NCI-H1975 xenografts are treated with either a single dose of vehicle or ganetespib, or with 5 daily doses of vehicle or ganetespib, in groups of 3 or 8, and harvested at various time points. Tumors are excised and flash frozen in liquid nitrogen for preparation of protein lysates or fixed in 10% neutral buffered formalin for immunohistochemistry. |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 685.8±57.0 °C at 760 mmHg |
| Flash Point | 368.5±32.1 °C |
| Exact Mass | 364.153534 |
| PSA | 96.33000 |
| LogP | 5.47 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Storage condition | -20℃ |