Home
Products
Danoprevir (ITMN-191)
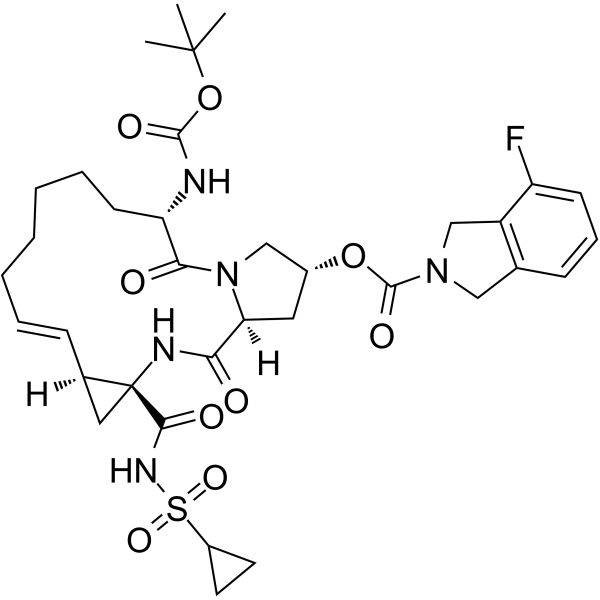


| Product Name | Danoprevir (ITMN-191) |
| Price: | Inquiry |
| Catalog No.: | CN00141 |
| CAS No.: | 850876-88-9 |
| Molecular Formula: | C35H46FN5O9S |
| Molecular Weight: | 731.83 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | O=C(OC(C)(C)C)N[C@H]1CCCCC/C=C[C@@H]2[C@](NC(=O)[C@H]3N(C1=O)C[C@@H](C3)OC(=O)N1Cc3c(C1)cccc3F)(C2)C(=O)NS(=O)(=O)C1CC1 |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Danoprevir is a NS3/4A protease inhibitor for hepatitis C virus (HCV) with IC50 of 0.2-3.5 nM. The inhibition effect on HCV genotypes 1A/1B/4/5/6 is approximately 10-fold higher than 2B/3A. |
| Target | IC50: 0.2-3.5 nM (NS3/4A protease) |
| In Vitro | In Huh7.5 cells transfected with chimeric recombinant virus, Danoprevir shows antiviral inhibition effects against HCV genotypes 1, 4 and 6 with IC50 of 2-3 nM, which are >100-fold lower than genotypes 2/3/5 (280-750 nM)[1]. Danoprevir (0.29 nM) inhibits the reference genotype 1 NS3/4A protease half-maximally, but a high dose of Danoprevir (10 μM) shows no appreciably inhibition in a panel of 79 proteases, ion channels, transporters, and cell surface receptors. Danoprevir remains bound to and inhibits NS3/4A for more than 5 hours after its initial association. Danoprevir (45 nM) eliminates a patient-derived HCV genotype 1b replicon from hepatocyte-derived Huh7 cells with an EC50 of 1.8 nM[2]. In HCV subgenomic replicon cell lines containing the individual mutations, V36M, R109K, and V170A substitutions confer little or no resistance to Danoprevir, but the R155K substitution confers a high level (62-fold increase) of resistance to Danoprevir[3]. |
| In Vivo | Danoprevir (30 mg/kg, p.o.) administered to rats or monkeys shows that its concentrations in liver 12 hours after dosing exceed the Danoprevir concentration required to eliminate replicon RNA from cells[2]. |
| Animal Admin | Pharmacokinetic properties are evaluated in rats and monkeys. Sprague-Dawley rats (three per time point) are administered a 30-mg/kg of body weight dose of ITMN-191 by oral gavage (a 6-mg/mL solution in water). Cynomolgus monkeys (two per time point) are administered a 30-mg/kg dose of ITMN-191 by oral gavage (a 3-mg/mL solution in water). For each species, terminal blood samples and the entire perfused liver are collected 1, 4, 8, 12, and 24 h after dose administration. Blood samples are collected in EDTA, processed for plasma by centrifugation at 5°C, and stored at −20°C until analysis is performed. Liver samples are snap-frozen and stored at −70°C until analysis is performed. Blank, standard, and unknown plasma samples and homogenized liver containing an internal standard (ITMN-191 analog) are treated with acidified acetonitrile and centrifuged to remove precipitated proteins. The density of liver tissue is taken into account to allow concentrations in both compartments to be expressed as weight per unit volume. The cleared supernatants are diluted 1:1 into high-performance liquid chromatography grade water and analyzed on a 4000 Q-trap liquid chromatography-tandem mass spectrometer fitted with the Turbo-Ionspray source operating in negative-ion mode. Analytes and internal standards are monitored using multiple-reaction-monitoring scans and calibrated with ABI Analyst software, version 1.4.2. The calibration standards ranges from 0.0169 ng/mL to 37.0 ng/mL and from 7.47 ng/mL to 5,440 ng/mL for the quantification of plasma samples and liver homogenates, respectively. Quadratic fitting with 1/x weighting is utilized where an R2 value of > 0.999 is achieved in both matrices. |
| Density | 1.4±0.1 g/cm3 |
| Exact Mass | 731.300049 |
| PSA | 199.37000 |
| LogP | 1.12 |
| Storage condition | -20℃ |