Home
Products
Obatoclax Mesylate (GX15-070)
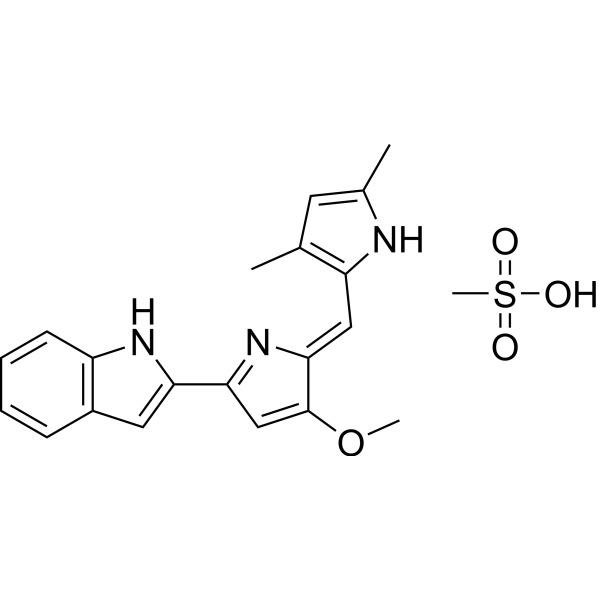


| Product Name | Obatoclax Mesylate (GX15-070) |
| Price: | Inquiry |
| Catalog No.: | CN00358 |
| CAS No.: | 803712-79-0 |
| Molecular Formula: | C20H19N3O.CH4O3S |
| Molecular Weight: | 413.49 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | CS(=O)(=O)O.COC1=CC(=N/C/1=Cc1[nH]c(cc1C)C)c1cc2c([nH]1)cccc2 |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Obatoclax is an antagonist of the BCL-2 family proteins. It binds to BCL-2 with a Ki of 220 nM. |
| Target | Bcl-2:220 nM (Ki) Bcl-xL:1-7 μM (Ki) Mcl-1:1-7 μM (Ki) Bcl-W:1-7 μM (Ki) Bcl-B:1-7 μM (Ki) Autophagy |
| In Vitro | Obatoclax (0, 0.5, 1, 2.5, 5, and 10 μM) effectively abrogates the growth of OCI-AmL3 cells, and similar results are seen in HL60, KG1, and U937 cells. Obatoclax also displays low-dose antiproliferative properties that are accompanied by a S/G2 cell cycle block. Obatoclax (10 μM) induces apoptosis proceeds through the intrinsic apoptotic pathway after neutralization of Mcl-1. Obatoclax synergizes with AraC and ABT-737 in inducing apoptosis in AmL cell lines. Obatoclax (250 nM) induces apoptosis and selectively inhibits colony formation of primary AmL cells[1]. Obatoclax induces cell death, with IC50 of 3.18 μM, 0.85 μM, and 0.76 μM for K1, BCPAP, and KTC-1 cells, respectively. Obatoclax also enhances cytotoxicity of Vemurafenib through inducing mixed cell death forms, loss of MOMP, suppression of mitochondrial respiration, and cellular glycolysis. Obatoclax regulates both the induction and degradation phases of authophagy, and promotes Mcl-1/Beclin-1 dependent autophagy in K1 cells[2]. Obatoclax inhibits cell proliferation and induces G1 cell-cycle arrest in a panel of human colorectal cancer cell lines, and the IC50 of cell proliferation at 72 h are 25.85, 40.69, and 40.01 nM for HCT116, HT-29, and LoVo cells, respectively. Obatoclax (0, 25, 50, 100, 200 nM) downregulates cyclin D1 to induce G1-phase arrest and consequent antiproliferation. Obatoclax (200 nM) targets cyclin D1 for proteasome-mediated degradation[3]. Obatoclax (500 nM, 1 μM) induces necrotic cell death and induces a block in autophagy, unrelated to cell death at 500 nM. Obatoclax localizes to lysosomes, affects lysosome structure and properties, but does not cause massive lysosomal permeabilization[4]. |
| In Vivo | The LY3009120 monotherapy or Obatoclax+Vemurafenib (20 mg/kg/day for combination) retards tumor growth more thoroughly in subcutaneous xenograft model of thyroid cancer[2]. Obatoclax (5 mg/kg, i.p.) effectively reduces tumor growth in mice[4]. |
| Cell Assay | HCT116, HT-29, and LoVo cells are seeded onto six-well plates at a density of 5×104/well, followed by treatment with obatoclax (0, 50, 100, 200 nM). The numbers of cells at 24, 48, and 72 h after obatoclax treatment are harvested, resuspended, stained with trypan blue, and then counted using a Neubauer chamber. |
| Animal Admin | Mice harboring [Pten, p53]thyr−/− tumors are treated with Obatoclax (5 mg/kg) via i.p. injection once every day for 6 days. Tumors are dissected and digested with Liberase. Single cell suspensions are incubated with 100 nM Lysotracker Deep Red for 30 minutes before flow cytometry analysis. |
| Exact Mass | 413.140930 |
| PSA | 115.92000 |
| LogP | 4.50730 |
| Storage condition | -20°C |