Home
Products
Simvastatin
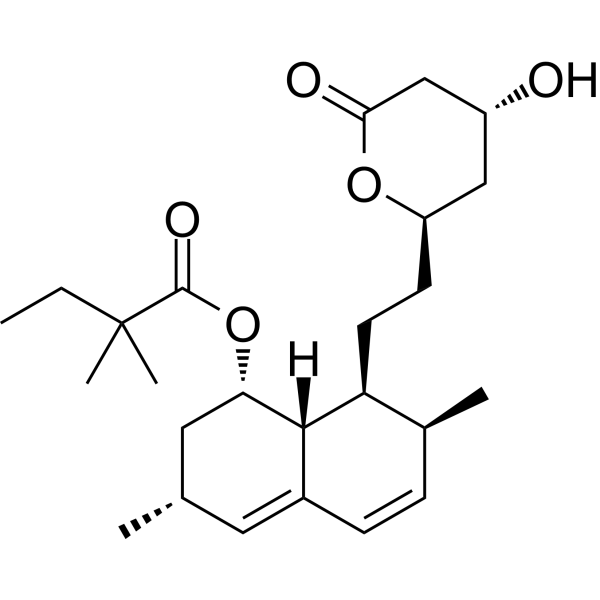


| Product Name | Simvastatin |
| Price: | $31 / 10mg |
| Catalog No.: | CN08663 |
| CAS No.: | 79902-63-9 |
| Molecular Formula: | C25H38O5 |
| Molecular Weight: | 418.56 g/mol |
| Purity: | >=98% |
| Type of Compound: | Diterpenoids |
| Physical Desc.: | Cryst. |
| Source: | From Penicillium Citrinum |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | CCC(C(=O)OC1CC(C)C=C2C1C(CCC1CC(O)CC(=O)O1)C(C=C2)C)(C)C |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Simvastatin (MK 733) is a competitive inhibitor of HMG-CoA reductase with a Ki of 0.2 nM. |
| Target | Ki: 0.2 nM (HMG-CoA reductase)[1] |
| In Vitro | Simvastatin needs to be activated by NaOH in EtOH treatment before use for cell assay. Simvastatin suppresses cholesterol synthesis in mouse L-M cell, rat H4II E cell, and human Hep G2 cell with IC50s of 19.3 nM, 13.3 nM and 15.6 nM, respectively[1]. Simvastatin causes a dose-dependent increase in serine 473 phosphorylation of Akt within 30 minutes, with maximal phosphorylation occurring at 1.0 µM. Simvastatin (1.0 μM) enhances phosphorylation of the endogenous Akt substrate endothelial nitric oxide synthase (eNOS), inhibits serum-free media undergo apoptosis and accelerates vascular structure formation[2]. Simvastatin shows anti-inflammatory effects, reduces anti-CD3/anti-CD28 antibody-stimulated proliferation of PB-derived mononuclear cells and synovial fluid cells from rheumatoid arthritis blood, as well as IFN-γ release at 10 μM. Simvastatin (10 μM) also blocks cell-mediated macrophage TNF-γ release induced via cognate interactions by appr 30%[3]. Simvastatin (5 μM) significantly reduces the expression of ABCA1 in astrocytes and neuroblastoma cells, the expression of apolipoprotein E in astrocytes, and increases cyclin-dependent kinase 5 and glycogen synthase kinase 3β expression in SK-N-SH cells[7]. |
| In Vivo | Simvastatin suppresses the conversion of radiolabeled acetate to cholesterol with an IC50 of 0.2 mg/kg via p.o. administration[1]. Simvastatin (4 mg/day, p.o. for 13 weeks) returns the cholesterol-induced increases in total cholesterol, LDL-cholesterol and HDL-cholesterol to normal level in rabbits fed an atherogenci cholesterol-rich diet[4]. Simvastatin (6 mg/kg) increases LDL receptor-dependent binding and the number of hepatic LDL receptors in rabbits fed a diet containing 0.25% cholesterol[5]. Simvastatin affects inflammation independent of its effect on plasma cholesterol level. Simvastatin (20 mg/kg/day) causes a 1.3-fold less macrophage content in lesions, and 2-fold less vascular cell adhesion molecule-1, interleukin-1beta, and tissue factor expression, companied by a 2.1-fold increases in lesional smooth muscle cell and collagen content in cynomolgus monkeys fed an atherogenic diet[6]. |
| Cell Assay | Primary human astrocytes from four different donors are prepared from tissue obtained from legally aborted fetuses. Primary human astrocytes and SK-N-SH neuroblastoma cell line (ATCC) are plated on 6-well plates and grown in DMEM supplemented with 5% or 8% fetal calf serum, respectively, at 37°C, 5% CO2 until 80% confluent. For measurement of gene expression levels at baseline, cells are just washed and RNA is prepared and assayed as outlined below. Baseline gene expression levels in astrocytes are measured in primary human astrocytes obtained from two donors. For experimental purposes, cells are incubated under serum-free conditions. Primary human astrocytes are obtained from four donors. Based on preliminary time-dependent studies, 48-h incubation is used for all of experiments. Based on the dose-response studies, a majority of our experiments are conducted using the following concentrations of active compounds: Simvastatin at 5 μM, pravastatin at 10 μM, mevalonate at 50 μM, and GGPP and FPP at 10 μM. Following incubation, cells are extensively washed to remove dead cells and cell debris and prepared for further analyses[7]. |
| Animal Admin | Monkeys[1] Thirty-nine adult male cynomolgus monkeys are initially fed a moderately atherogenic diet containing 0.28 mg cholesterol per calorie of diet, with 16.7% from protein, 45.1% from lipids, and 38.1% from carbohydrates. After consuming the atherogenic diet for 3 months, the monkeys are divided into 3 groups (n=13 each) that are equivalent in their total plasma cholesterol (TPC), LDL-C, and HDL cholesterol (HDL-C) concentrations; these groups consume the atherogenic diet and receive statin (or control) treatment for an additional 15 months. Control monkeys are fed the atherogenic diet with no added statins. Prava-treated monkeys have 40 mg Prava/kg body wt per day added to the atherogenic diet. Simvastatin-treated monkeys consumed 20 mg Simvastatin/kg body wt per day[1]. |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 564.9±50.0 °C at 760 mmHg |
| Flash Point | 184.8±23.6 °C |
| Exact Mass | 418.271912 |
| PSA | 72.83000 |
| LogP | 4.41 |
| Vapour Pressure | 0.0±3.5 mmHg at 25°C |