Home
Products
Volasertib (BI 6727)
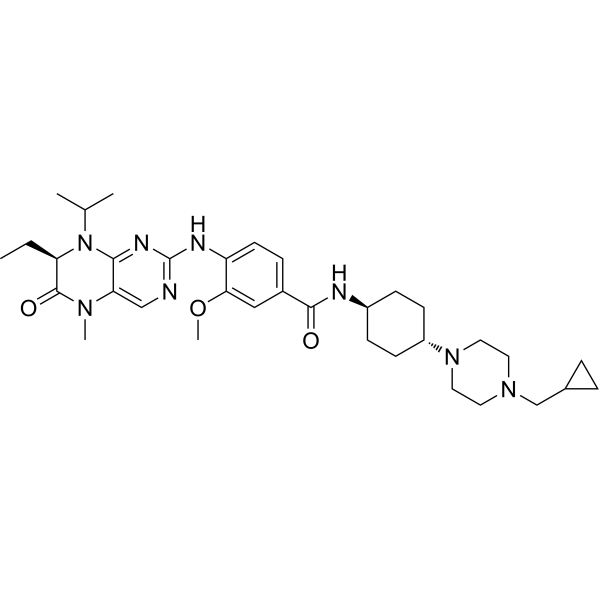


| Product Name | Volasertib (BI 6727) |
| Price: | Inquiry |
| Catalog No.: | CN00364 |
| CAS No.: | 755038-65-4 |
| Molecular Formula: | C34H50N8O3 |
| Molecular Weight: | 618.81 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | CC[C@H]1N(C(C)C)c2nc(ncc2N(C1=O)C)Nc1ccc(cc1OC)C(=O)N[C@@H]1CC[C@H](CC1)N1CCN(CC1)CC1CC1 |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Volasertib is a highly potent Polo-like kinase 1 (PLK1) inhibitor with an IC50 of 0.87 nM, as well as the two closely related kinases PLK2 and PLK3 with IC50s of 5 and 56 nM, respectively. |
| Target | PLK1:0.87 nM (IC50) PLK2:5 nM (IC50) PLK3:56 nM (IC50) |
| In Vitro | Volasertib is potent against HeLa and Caski cells with IC50 values of 0.02 μM and 2.02 μM, respectively. Volasertib (0.03 μM) induces cell cycle arrest at G2/M Phase in cervical cancer cells. Volasertib (0.003-0.03 μM) induces apoptosis in HeLa cells, and Volasertib (0.3-3 μM) results in Caski cell apoptosis. Volasertib (1, 3 μM or 0.01,0.03 μM) augments the fluorescent intensity of DHE in Caski and HeLa cells in a dose-dependent manner[1]. Volasertib shows inhibitory activities against the proliferation of all 40 cell lines tested, with a mean half-maximal growth inhibitory concentration of 313 nM (range: 4-5000 nM)[2]. Volasertib inhibits proliferation of multiple cell lines derived from various cancer tissues, including carcinomas of the colon (HCT 116, EC50=23 nM) and lung (NCI-H460, EC50=21 nM), melanoma (BRO, EC50=11 nM), and hematopoietic cancers (GRANTA-519, EC50=15 nM; HL-60, EC50=32 nM; THP-1, EC50=36 nM and Raji, EC50=37 nM) with EC50 values of 11 to 37 nM. Volasertib (100 nM) causes G2-M arrest and induces apoptosis in NCI-H460 cells[3]. |
| In Vivo | Volasertib (15 mg/kg, i.p.) potentiates the activity of cisplatin to inhibit xenograft tumor growth of cervical cancer cells in nude mice[1]. Volasertib (70 mg/kg, p.o. once a week or 10 mg/kg, p.o. daily) significantly delays tumor growth in a non-small cell lung carcinoma xenograft model. In addition, Volasertib (15 mg/kg, i.v.) markedly suppresses tumor growth and is well tolerated[3]. |
| Cell Assay | Cells are firstly seeded into a 96-well plate at a density of 5000 cells per well, and incubated with drugs in three parallel wells for 72 hr. Then MTT is added to each well at a final concentration of 0.5 mg/mL. After incubation for 4 hr, formazan crystals are dissolved in 100 μL of DMSO, and absorbance at 570 nm is measured by plate reader. The concentrations required to inhibit growth by 50% (IC50) are calculated from survival curves[1]. |
| Animal Admin | Female BomTac: NMRI-Foxn1nu mice are grafted s.c. with 2×106 HCT 116 human colon carcinoma cells (ATCC CCL-247), 1×106 NCI-H460 non-small cell lung cancer cells (ATCC HTB-177), or CXB1 human colon carcinoma tumor pieces derived from patient material by serial transplantation in nude mice. When tumors have reached a volume of appr 50 to 100 mm3, animals are randomized into treatment and control groups of 10 mice each. Volasertib is formulated in hydrochloric acid (0.1 N), diluted with 0.9% NaCl, and injected i.v. into the tail vein at the indicated dose and schedule. For oral treatment, Volasertib is resuspended in 0.5% Natrosol 250 hydroxyethyl-cellulose and given intragastrally via gavage needle. An administration volume of 10 mL per kilogram of body weight is used for both administration routes. Tumor volumes are determined thrice a week using a caliper. The results are converted to tumor volume (mm3) by the formula length×width2×π/6. The weight of the mice is determined as an indicator of tolerability on the same days. Median tumor volumes on the last day of the experiment are used to calculate treated versus control values (= tumor volumetreated mice ×100/tumor volumecontrol mice). |
| Density | 1.3±0.1 g/cm3 |
| Exact Mass | 618.400574 |
| PSA | 109.66000 |
| LogP | 2.44 |
| Storage condition | -20°C |