Home
Products
Tyramine
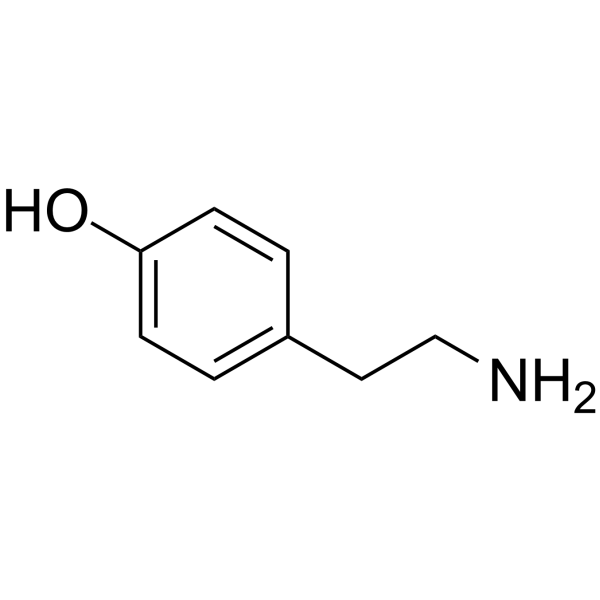


| Product Name | Tyramine |
| Price: | Inquiry |
| Catalog No.: | CN00964 |
| CAS No.: | 51-67-2 |
| Molecular Formula: | C8H11NO |
| Molecular Weight: | 137.18 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | The herbs of Uncaria rhynchophylla. |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | NCCc1ccc(cc1)O |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Tyramine is an indirectly acting sympathomimetic amine that releases norepinephrine from presynaptic nerve terminals. |
| Target | Human Endogenous Metabolite |
| In Vitro | Tyramine is an indirectly acting sympathomimetic amine that releases norepinephrine from presynaptic nerve terminals[1]. Tyramine potentiates the postsynaptic inhibitory effects of norepinephrine on the firing of cortical neurones in the rat[2]. Although tyramine exists in three isomerie forms, the orthoisomer and metaisomers are present in very low concentrations and it is the paraisomer that is implied when reference is made to tyramine. The tyramines are generally present in nanogram/gram concentrations in brain tissue, but they have a very rapid turnover. The CNS role of tyramine is unclear, it has been suggested that it is involved in the modulation of dopamine synthesis and may also regulate norepinephrine turnover[3]. |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 275.1±23.0 °C at 760 mmHg |
| Flash Point | 141.3±13.3 °C |
| Exact Mass | 137.084061 |
| PSA | 46.25000 |
| LogP | 1.38 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Storage condition | Refrigerator, Under Inert Atmosphere |