Home
Products
Sotrastaurin
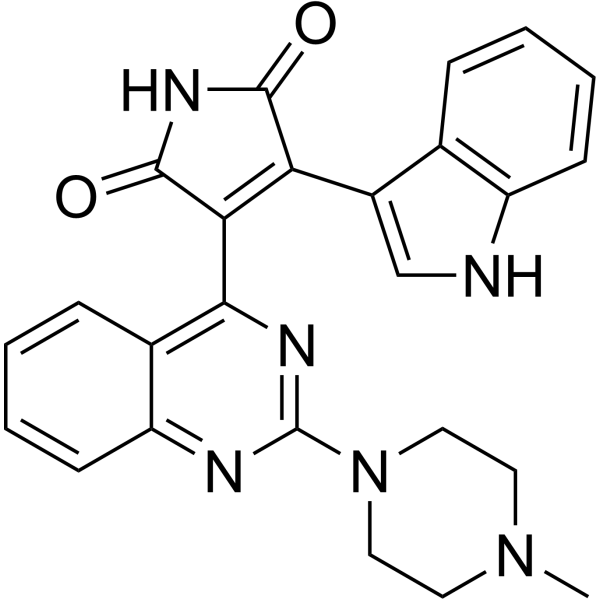


| Product Name | Sotrastaurin |
| Price: | Inquiry |
| Catalog No.: | CN00404 |
| CAS No.: | 425637-18-9 |
| Molecular Formula: | C25H22N6O2 |
| Molecular Weight: | 438.48 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | CN1CCN(CC1)c1nc2ccccc2c(n1)C1=C(C(=O)NC1=O)c1c[nH]c2c1cccc2 |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Sotrastaurin is a potent pan-PKC inhibitor, with Kis of 0.22 nM, 0.64nM, 0.95 nM, 1.8 nM, 2.1 nM and 3.2 nM for PKCθ, PKCβ, PKCα, PKCη, PKCδ and PKCε, respectively. |
| Target | PKCθ:0.22 nM (Ki) PKCβI:0.64 nM (Ki) PKCα:0.95 nM (Ki) PKCη:1.8 nM (Ki) PKCδ:2.1 nM (Ki) PKCε:3.2 nM (Ki) |
| In Vitro | In cell-free kinase assays Sotrastaurin (AEB071) inhibits PKC, with Ki values in the subnanomolar to low nanomolar range. When Sotrastaurin is tested on a selected panel of kinases, the only enzyme on which Sotrastaurin displays an IC50value below 1 μM is glycogen synthase kinase 3β[1]. Sotrastaurin (AEB071) inhibits p-MARCKS, a PKC substrate, and pS6 in all the cell lines, independently of the mutational status. There is a slight inhibition of pERK at lower doses also in the GNA11 mutant cells, but not in the WT cells at any concentrations. This is consistent with previous reports indicating that Sotrastaurin inhibits ERK1/2 phosphorylation in GNAQ mutant cell lines[2]. |
| In Vivo | The combination therapy results in a significantly enhanced reduction in tumor volume when compared to either Sotrastaurin (AEB071) or BYL719 alone (p=0.049 vs. BYL719 and p=0.022 vs. Sotrastaurin at day 26). There is even a greater effect when compared to vehicle control (p=0.016)[2]. Sotrastaurin (STN) treatment of liver donors and orthotopic liver transplantation (OLT) recipients (Gr.I) or of OLT recipients alone (Gr.II) prolongs animal survival, as 9 out of 10 rats in Gr. I, and 6 out of 6 rats in Gr.II survive >14 days. In contrast, only 4 out of 10 control OLT recipients remain alive at day 14 (p<0.01)[3]. |
| Cell Assay | Cells are plated in a 96-well plate and treated with Sotrastaurin, BYL719 or DMSO at indicated concentrations for a period of 5 days. Viability is assessed using Cell Counting Kit. The Combination Index values are calculated using the CompuSyn software. Briefly explained, the plots generated by the CompuSyn software demonstrate the Y-axis combination index values, where CI<1, =1, and >1 indicate synergism, additive effect, and antagonism, respectively. The X-Axis represents the fractional activity, which reflects the fraction of cells inhibited by the treatments relative to vehicle control. For combination index studies, the concentrations tested included Sotrastaurin (0, 125, 250, 500, 1000 nM) and BYL719 (0, 250, 500, 1000, 2000 nM)[2]. |
| Animal Admin | Mice[2] 6-8 week nu/nu SCID female mice bearing subcutaneously injected 92.1 tumors (7 mice/group) of 100mm3 diameter are treated with vehicle, Sotrastaurin (80mg/kg/d) TID and or BYL719 orally (50mg/kg/d) QD as single agents and in combination, 5 days/week for 2 weeks. After 2 weeks, two animals from each group are sacrificed and tumors are collected to analyze for Western blot. For Omm1 xenogratfs, 6-8 weeks athymic female mice bearing subcutaneously injected Omm1 tumors (7 mice/group) of 100 mm3 diameter are treated with vehicle, Sotrastaurin (80mg/kg/d) TID and or BYL719 orally (50mg/kg/d) QD as single agents and in combination, 5 days/week for 3 weeks. Tumors are homogenized with grinding resins kits. Tumors are collected to analyze for H&E, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Tumors are measured every 2 to 3 days with calipers, and tumor volumes are calculated. Toxicity is monitored by weight loss. Rats[3] Male Sprague-Dawley (SD) rats (230-250g) are used throughout.Livers from SD rats are stored at 4C in UW solution for 30h, and then transplanted to SD rats with revascularization. Sotrastaurin (30mg/kg b.i.d. via oral gavage) is used in two treatment protocols. In Gr. I (n=10), liver Sotrastaurin is given to liver donors (90min prior to organ harvest) and OLT recipients (90min prior to the transplant, and for three days post-OLT). In Gr. II (n=6), Sotrastaurin is administered to OLT recipients only (according to Gr. I protocol). Gr. III controls are treated with PBS (n=10). OLT survival is assessed at day 14. Separate cohorts in Gr. I (n=3-4/gr) are sacrificed at 6h and 24h; OLT and peripheral blood samples are collected for analyses. |
| Density | 1.4±0.1 g/cm3 |
| Exact Mass | 438.180420 |
| PSA | 97.71000 |
| LogP | 2.56 |
| Storage condition | -20℃ |