Home
Products
PD98059
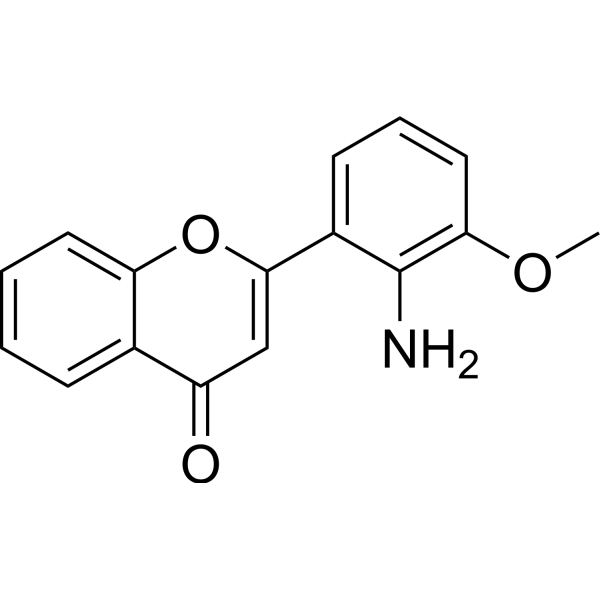


| Product Name | PD98059 |
| Price: | Inquiry |
| Catalog No.: | CN00547 |
| CAS No.: | 167869-21-8 |
| Molecular Formula: | C16H13NO3 |
| Molecular Weight: | 267.28 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | COc1cccc(c1N)c1cc(=O)c2c(o1)cccc2 |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | PD98059 is a potent, selective and cell-permeable MEK1 and MEK2 inhibitor with IC50s of 4 μM and 50 μM respectively. |
| Target | MEK:5 μM (IC50) Autophagy |
| In Vitro | Concentrations of PD98059 of ≤20 μM are not cytotoxic to cultured MCF10A, MCF10A-Neo, and MCF10A-NeoT cells. However, PD98059 is weakly cytostatic to all three lines at concentrations of ≥10 μM. Treatment of MCF10A-Neo and MCF10A-NeoT cultures with concentrations of PD98059 up to 20 μM for 2-22 hr does not alter the total ERK content. However, treatment with PD98059 does result in concentration-dependent reductions in the dually phosphorylated forms of ERK1 and ERK2. Within 2 hr of a 10-μM treatment, phosphorylated ERK contents are reduced ~74% and ~86% in MCF10A-Neo and MCF10A-NeoT cultures, respectively (IC50=1 μM). Within 22 hr of treatment, phosphorylated ERK forms are almost completely eliminated in both cell lines[1]. PD98059 (PD 098059) prevents the activation of MAPKK1 by Raf or MEK kinase in vitro at concentrations (IC50=2-7 μM). PD98059 inhibits both the activation and phosphorylation of MAPKK1 in vitro by either c-Raf or MEK kinase with IC50 values of 4±2 μM. Incubation of Swiss 3T3 cells with PD98059 (50 μM) suppressed by 80-90% the activation of MAPKK induced by each agonist, but the activation of c-Raf is enhanced 2-3-fold[2]. |
| In Vivo | The treatment of mice with PD98059 significantly reduces the level of p-ERK1/2. Moreover, a significant increase in the phospho-p38 expression is observed in Zymosan-treated mice at 18 h after Zymosan administration compared to the sham-operated mice. The treatment with PD98059 significantly reduces the p38 expression[3]. Repeated treatment with PD98059 attenuates mechanical allodynia measured by the von Frey test three (18.0 g±0.8, n=10) and seven (20.21 g±0.67, n=26) days after CCI in comparison to the vehicle-treated CCI-exposed rats (15.1 g±1.3, n=7 and 14.21 g±0.44, n=28, respectively). Repeated injection of PD98059 diminishes thermal hyperalgesia, as is evaluated by the cold plate test, three (17.5 s±2.1, n=10) and seven (25.54 s±1.03, n=26) days following CCI compared to vehicle-treated CCI-exposed rats (11.5 s±1.8, n=7 and 11.4 s±0.88, n=28, respectively)[4]. |
| Cell Assay | The MCF10A, MCF10A-Neo, and MCF10A-NeoT cell lines are used. Subconfluent cultures are treated with PD98059 (0-100 μM). Viability of cells after treatment is assessed by ability to exclude trypan blue. Cultures earmarked for RNA isolation are washed twice with phosphate-buffered saline (2.7 mM KCl, 1.5 mM KH2PO4, 137 mM NaCl, 8 mM Na2HPO4, pH 7.2) at harvesting and stored at -80°C[1]. |
| Animal Admin | Mice[3] Male CD mice (20-22 g) are randomly allocated into the following groups: 1. Zymosan+DMSO group. Mice are treated intraperitoneally (i.p.) with Zymosan (500 mg/kg, suspended in saline solution) and with the vehicle for PD98059 (10% DMSO, v/v) i.p. 1 and 6 h after Zymosan administration (N=10). 2. PD98059 group. Identical to the Zymosan+DMSO group but are administered PD98059 (10 mg/kg, i.p. bolus) at 1 and 6 h after Zymosan (N=10) instead of DMSO. 3. Sham+DMSO group. Identical to the Zymosan+DMSO group but are administered saline solution instead of Zymosan (N=10). 4. Sham+PD98059 group. Identical to Sham+DMSO group, except for the administration of PD98059 (10 mg/kg i.p. bolus) 1 and 6 h after saline administration (N=10). Rats[4] The rats (male Wistar, 300-350 g) are used. The PD98059 (2.5 μg/5 μL, i.t.) is single or repeated preemptively administered 16 h and 1 h before CCI and then once daily for 7 days. The Vehicle-treated CCI-exposed rats receive 75% DMSO according to the same schedule. There is no significant difference in pain behavior between no-treated and V(DMSO)-treated CCI-exposed rats. This method of PD98059 or vehicle administration is used throughout the study and is referred to in the text as “repeated administration”. At day 7th after CCI 30 min after PD98059 administration tactile allodynia is measured using von Frey test and thermal hyperalgesia is conducted using cold plate test. Additionally, at day 7th after CCI the vehicle-treated and PD98059-treated rats receive a single i.t. vehicle, Morphine (2.5 μg/5 μL) or Buprenorphine (2.5 μg/5 μL) injection 30 min after PD98059, and then 30 min later the von Frey and/or cold plate tests are repeated. Since the dose of morphine 2.5 μg/5 μL in naive rats produces maximal analgesic effect in tail-flick test. Lower dose of Morphine are used for co-administration experiments, so that observing the possible enhancement of opioid effectiveness. The vehicle-treated and PD98059-treated naive rats (uninjured rats) receive a single i.t. vehicle, Morphine (0.5 μg/5 μL) or Buprenorphine (2.5 μg/5 μL) injection 30 min after PD98059, and then 30 min later the tail flick test is performed. |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 453.1±45.0 °C at 760 mmHg |
| Flash Point | 221.9±25.0 °C |
| Exact Mass | 267.089539 |
| PSA | 65.46000 |
| LogP | 2.43 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Storage condition | −20°C |