Home
Products
Entospletinib (GS-9973)
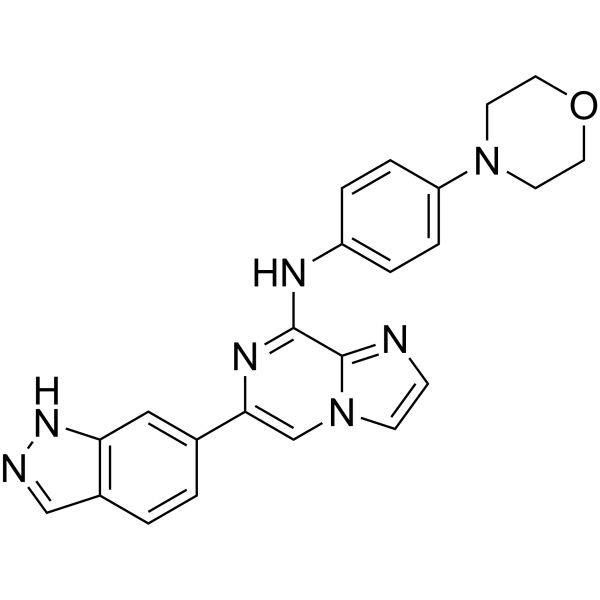


| Product Name | Entospletinib (GS-9973) |
| Price: | Inquiry |
| Catalog No.: | CN00290 |
| CAS No.: | 1229208-44-9 |
| Molecular Formula: | C23H21N7O |
| Molecular Weight: | 411.46 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | O1CCN(CC1)c1ccc(cc1)Nc1nc(cn2c1ncc2)c1ccc2c(c1)[nH]nc2 |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Entospletinib (GS-9973) is an orally bioavailable, selective?Syk inhibitor with an IC50 of 7.7 nM. |
| Target | IC50: 7.7 nM (Syk) |
| In Vitro | Entospletinib (GS-9973) shows good bidirectional permeability across Caco-2 cell monolayers in vitro. In cells, Entospletinib (GS-9973) also shows excellent selectivity for Syk, and potently inhibits BCR-mediated activation and proliferation of B-cells as well as immune-complex-stimulated cytokine production in monocytes[1]. The combination of idelalisib and Entospletinib (GS-9973) synergistically inhibits CLL cell viability and further disrupts chemokine signaling[2]. |
| In Vivo | Entospletinib (GS-9973) (1 mg/kg, p.o.) shows moderate to high bioavailability in rat and dog. In a rat collagen-induced arthritis model, Entospletinib (GS-9973) (1-10 mg/kg, p.o.) significantly inhibits ankle inflammation. Moreover, Entospletinib (GS-9973) also shows disease-modifying activity in multiple histological measurements, including inhibition of pannus formation, cartilage damage, bone resorption, and peritosteal bone formation with ED50 ranging from 1.2 to 3.9 mg/kg[1]. |
| Cell Assay | Functional impact on cellular Flt3 activity is determined by measuring compound inhibition of MV-4-11 cell proliferation. A total of 104 cells are diluted in RPMI medium containing 10% FBS in 96-well flat-bottomed tissue culture plates and incubated with compound dilutions for 72 h at 37°C. Alamar blue (10%) is added to the cells, which are incubated for an additional 12-18 h at 37°C, and inhibition of the relative cell numbers is determined by spectrophotometer readings at 570/600 nm. |
| Animal Admin | Female Lewis rats (mean mass 178 g, eight per group for collagen arthritis, four per group for normal controls) are anesthetized with isoflurane and injected with 300 μL of Freund’s incomplete adjuvant containing 2 mg/mL bovine type II collagen at the base of the tail and two sites on the back on days 0 and 6. Oral dosing (bid at 12 h intervals) is performed on arthritic days 0-7 with vehicle (Cremophor/ethanol/saline), Entospletinib (GS-9973) (1, 3, or 10 mg/kg), or the reference compound dexamethasone (Dex; 0.075 mg/kg) administered daily (qd). Rats are terminated on arthritis day 16. Efficacy evaluation is based on animal body masses, daily ankle caliper measurements, ankle diameters expressed as the area under the curve (AUC), terminal hind paw masses, and histopathologic evaluation of ankles and knees. PK is measured from plasma samples taken 0, 2, 4, 8, 12, and 24 h post last dose. The paws are fixed in formalin and processed for hemotoxylin (H) and eosin (E) microscopy. H and E sections are scored for bone resorption as follows: (0) normal; (0.5) normal on low magnification but have the earliest hint of small areas of resorption in the metaphysis with no resorption in the tarsal bones; (1) (minimal) small definite areas of resorption in distal tibial trabecular or cortical bone or in the tarsal bones, not readily apparent on low magnification, rare osteoclasts; (2) (mild) more numerous areas (<25% loss of bone in growth plate area) of resorption in distal tibial trabecular or cortical bone and tarsals apparent on low magnification, osteoclasts more numerous; (3) (moderate) obvious resorption of medullary trabecular and cortical bone without full thickness defects in both distal tibial cortices, loss of some medullary trabeculae with 26-50% loss across the growth plate and cortices, some loss in tarsal bones, lesion apparent on low magnification, osteoclasts more numerous; (4) (marked) full or nearly full thickness defects in both distal tibial cortices, often with distortion of the profile of the remaining cortical surface, marked loss of medullary bone of distal tibia (50-100% loss across the growth plate area and cortices and up to 50% loss in small tarsals if minor in tibia), numerous osteoclasts, minor to mild resorption in smaller tarsal bones; (5) (severe) full thickness defects in both distal tibial cortices with >75% loss across the growth plate and both cortices and >50% loss in tarsals, often with distortion of the profile of the remaining cortical surface, marked loss of medullary bone of distal tibia, numerous osteoclasts. Osteoclast counts (5400× fields) are performed on the ankles in the areas of greatest bone resorption. |
| Density | 1.5±0.1 g/cm3 |
| Exact Mass | 411.180756 |
| PSA | 86.60000 |
| LogP | 3.74 |
| Storage condition | -20℃ |