Home
Products
Apitolisib (GDC-0980)
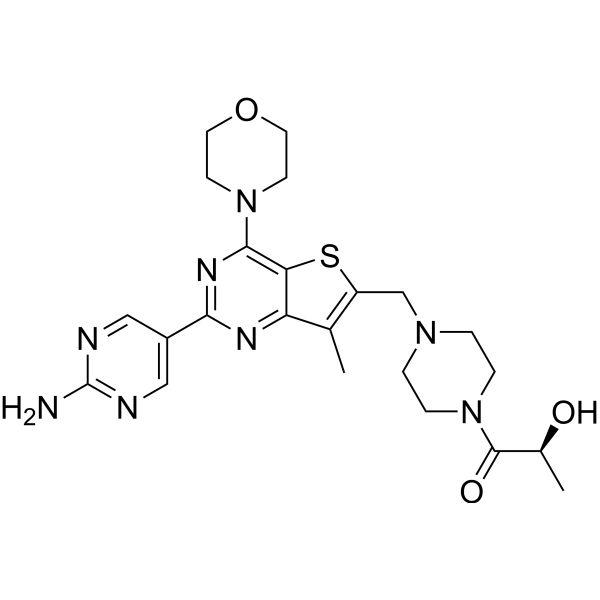


| Product Name | Apitolisib (GDC-0980) |
| Price: | Inquiry |
| Catalog No.: | CN00311 |
| CAS No.: | 1032754-93-0 |
| Molecular Formula: | C23H30N8O3S |
| Molecular Weight: | 498.6 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | O=C(N1CCN(CC1)Cc1sc2c(c1C)nc(nc2N1CCOCC1)c1cnc(nc1)N)[C@@H](O)C |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | Apitolisib (GDC-0980) is a selective, potent, orally bioavailable Class I PI3 kinase and mTOR kinase (TORC1/2) inhibitor with IC50s of 5 nM/27 nM/7 nM/14 nM for PI3Kα/PI3Kβ/PI3Kδ/PI3Kγ, and with a Ki of 17 nM for mTOR. |
| Target | PI3Kα:5 nM (IC50) PI3Kβ:27 nM (IC50) PI3Kδ:7 nM (IC50) PI3Kγ:14 nM (IC50) mTOR:17 nM (Ki) TORC1 TORC2 |
| In Vitro | Apitolisib (GDC-0980) is remarkably selective for several other members of the closely related PIKK family kinases: C2alpha IC50=1300 nM; C2beta IC50=7 94 nM; VPS34 IC50=2000 nM; PI4Kalpha >10 μM; PI4Kbeta >10 μM; DNA-PK Kiapp=623 nM, respectively[1]. A recent study shows that Apitolisib (GDC-0980) reduces cancer cell viability by inhibiting cell-cycle procession and inducing apoptosis with most potency in prostate (IC50 < 200 nM 50%), <500 nM 100%), breast (IC50 <200 nM 37%, <500 nM 78%) and NSCLC lines (IC50 <200 nM 29%, <500 nM 88%) and less potency in pancreatic (IC50 <200 nM 13%, <500 nM 67%) and melanoma cell lines (IC50 <200 nM 0%, <500 nM 33%)[2]. |
| In Vivo | Apitolisib (GDC-0980) (1 mg/kg, p.o.) demonstrats significant efficacy in mouse xenografts and is currently in phase I clinical trials for cancer. Clearance and PPB are low, and Apitolisib (GDC-0980) shows dose-proportional exposure from 5 mg/kg dosed in PEG to 50 mg/kg dosed in suspension in MCT, a finding attributed partially to the compound’s good solubility[1]. Apitolisib (GDC-0980) (5 mg/kg, p.o.) results in greater than 50% TGI in 15 of the 20 xenograft models. The difference in tumor response to Apitolisib (GDC-0980) treatment correlates with the duration of knockdown of pAkt/tAkt[2]. |
| Cell Assay | Three hundred and eighty-four-well plates are seeded with 2,000 cells/well in a volume of 54 μL per well followed by incubation at 37°C under 5% CO2 overnight (appr 16 hours). Compounds are diluted in dimethyl sulfoxide to generate the desired stock concentrations then added in a volume of 6 μL per well. All treatments are tested in quadruplicate. After 4 days incubation, relative numbers of viable cells are estimated using CellTiter-Glo and total luminescence is measured on a Wallac Multilabel Reader. The concentration of drug resulting in 50% inhibition of cell viability (IC50) or 50% maximal effective concentration (EC50) is determined using Prism software. For cell lines that failed to achieve an IC50 the highest concentration tested (20 μM) is listed. |
| Animal Admin | Human prostate cancer PC3 cells are resuspended in Hank’s Balanced Salt Solution and 3×106 cells implanted subcutaneously into the right hind flank of athymic nu/nu (nude) mice. Tumors are monitored until they reach a mean tumor volume of 150-200 mm3 prior to the initiation of dosing. MCF7.1 cells resuspended in a 1:1 mixture of Hank’s Buffered Salt Solution and Matrigel Basement Membrane Matrix are 5×106 subcutaneously implanted into the right hind flank of athymic nu/nu (nude) mice. Prior to cell inoculation, 17β-estradiol (0.36 mg/pellet, 60-day release, no. SE-121) are implanted into the dorsal shoulder blade area of each nude mouse. After implantation of cells, tumors are monitored until they reach a mean tumor volume of 250-350 mm3 prior to initiating dosing. Compound 2 is dissolved in 0.5% methylcellulose with 0.2% Tween-80 (MCT). Female nude (nu/nu) mice that are 6-8 weeks old and weighed 20-30 g are obtained from Charles River Laboratories. Tumor bearing mice are dosed daily for 14-21 days depending on the xenograft model with 100 μL of vehicle (MCT) or test agent orally. |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 718.6±70.0 °C at 760 mmHg |
| Flash Point | 388.4±35.7 °C |
| Exact Mass | 498.216156 |
| PSA | 162.80000 |
| LogP | 0.45 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Storage condition | -20℃ |